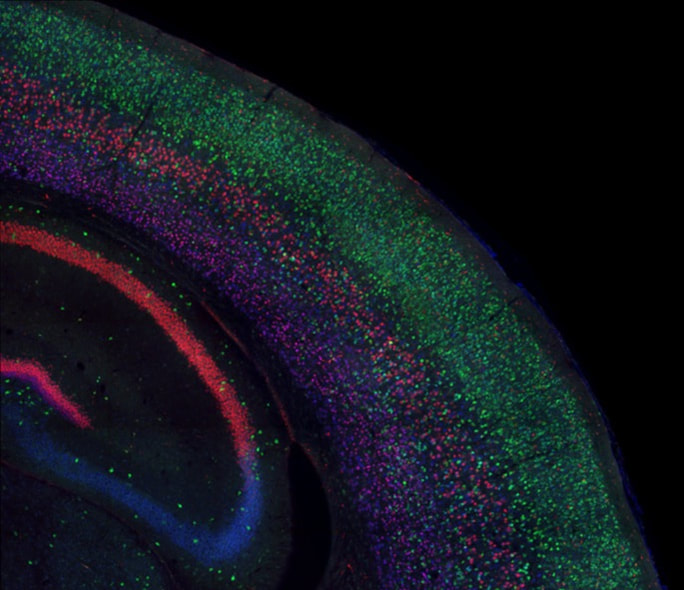
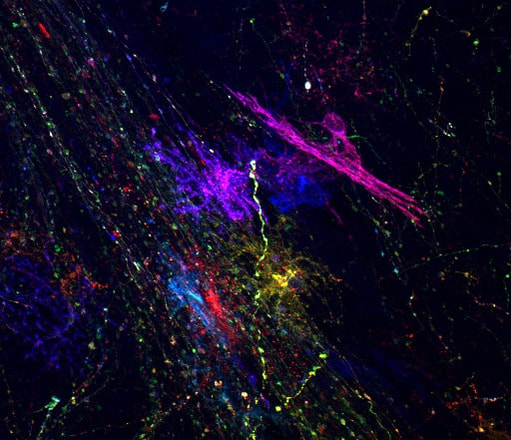
Fate Specification of Neural Stem Cells
One of the fundamental challenges of developmental neurobiology is to understand how an astoundingly diverse array of distinct cell types are produced and functionally connected, enabling us to sense, think and act. During early brain development, neural stem cells generate different types of neurons, as well as glia like astrocytes and oligodendrocytes. A central question in brain development has been how all of these different cell types are specified from the pool of uncommitted neural stem cells.
Our lab uses mouse models to study mechanisms of cell fate specification during embryonic brain development. We employ a combination of genetics, in utero electroporation, cell and molecular biology, and microscopy to understand cell lineage decisions in the normal and diseased brain.
Our lab uses mouse models to study mechanisms of cell fate specification during embryonic brain development. We employ a combination of genetics, in utero electroporation, cell and molecular biology, and microscopy to understand cell lineage decisions in the normal and diseased brain.
Specification of Neuron SubtypesDevelopment | (2019) March 7;146(5).
Lmx1a Drives Cux2 Expression in the Cortical Hem Through Activation of a Conserved Intronic Enhancer Fregoso SP, Dwyer BE, Franco SJ. Neuron | (2015) May 20;86(4):1091-9. Lineage Tracing Using Cux2-Cre and Cux2-CreERT2 Mice. Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, Zeng H, Franco SJ, Müller U. Neuron | (2013) Jan 9;77(1):19-34. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Franco SJ, Müller U. Science | (2012) Aug 10;337(6095):746-9. Fate-restricted neural progenitors in the mammalian cerebral cortex. Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Müller U. |
Specification of OligodendrocytesDevelopmental Biology | (2019) August 1;452(1):55-65.
Loss of Shh Signaling in the Neocortex Reveals Heterogeneous Cell Recovery Responses from Distinct Oligodendrocyte Populations Winkler CC, Franco SJ. Journal of Neuroscience | (2018) June 6;38(23):5237-5250. The Dorsal Wave of Neocortical Oligodendrogenesis Begins Embryonically and Requires Multiple Sources of Sonic Hedgehog Winkler CC, Yabut OR, Fregoso SP, Gomez HG, Dwyer BE, Pleasure SJ, Franco SJ. |