Neuronal Migration: Integrating New Neurons
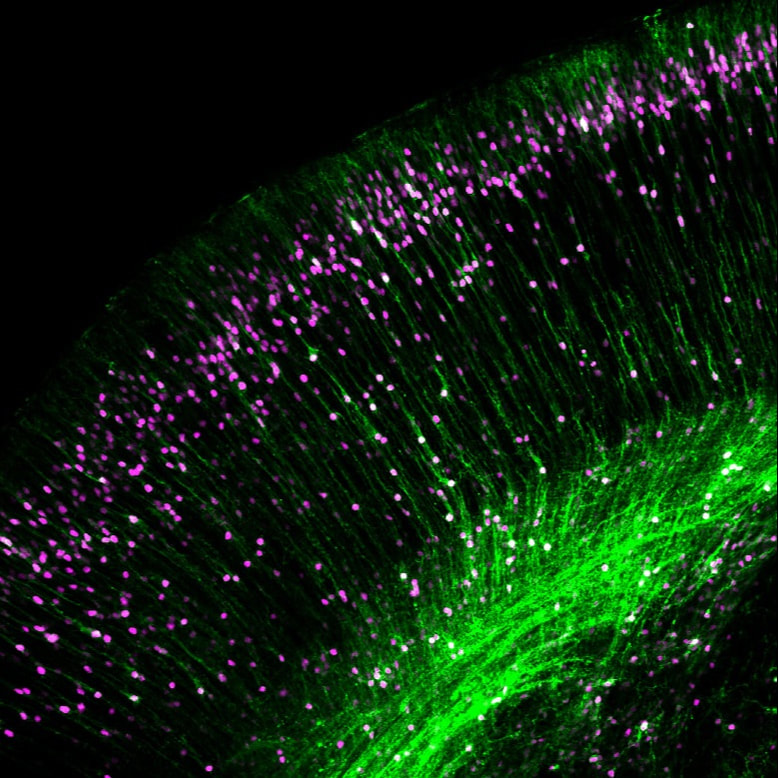
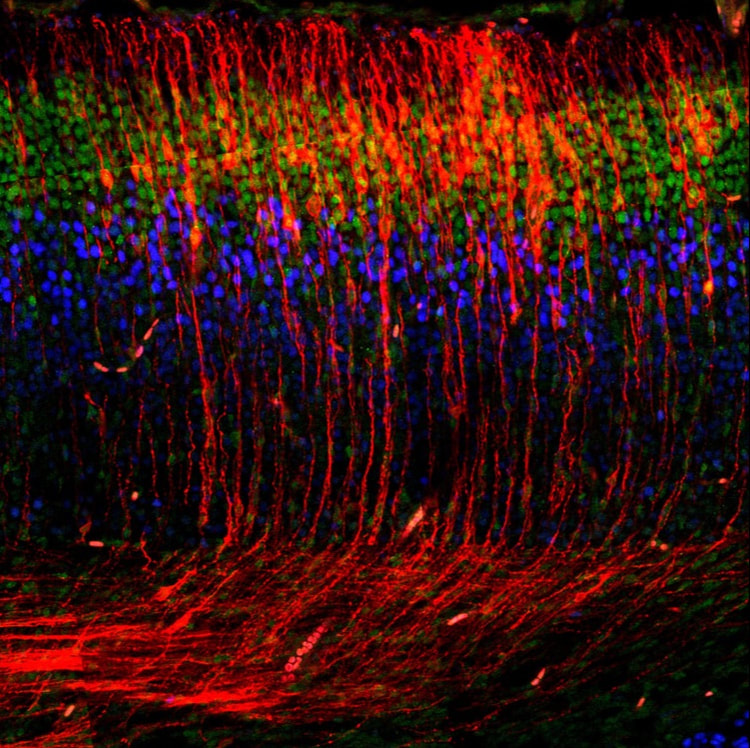
One of the most prominent anatomical features of the neocortex is its laminar organization, in which neurons with similar properties are segregated into specific cell layers that allow connections to be made efficiently. Importantly, none of the cortical cell types are actually generated locally within the cerebral cortex proper, but are made in distant germinal zones from where they migrate into the cortex. Therefore, the precise organization of the cerebral cortex depends on coordinated cycles of cell-fate specification, neurogenesis and neuronal migration. One of the best-known signaling molecules required for neuronal migration and cortical layer formation is Reelin. Mutations in Reelin signaling cause severe cortical abnormalities in humans and mice, including lissencephaly and disorganized layers. Reelin is a secreted glycoprotein that binds to receptors on the surface of migrating neurons, thereby inducing phosphorylation of the cytoplasmic adaptor protein Dab1. This event recruits several downstream signaling molecules, but the cellular functions that are regulated by these effectors are not well understood. While it is generally accepted that Reelin signaling regulates neuronal migration, contrasting models have been proposed for the cellular mechanisms of how Reelin controls cell motility and layer formation. For example, Reelin has been proposed to act as a chemoattractant, repellent, stop or detachment signal for migrating neurons. This controversy is compounded by the fact that neuronal migration is actually the sum of several distinct types of cell motility, each of which is likely to be controlled by different signaling pathways. Furthermore, different subtypes of projection neurons use distinct modes of migration depending on their target destination.
Our lab has helped define the cellular mechanisms of Reelin function in cortical lamination and elucidate the molecular pathway downstream of Reelin signaling during neuronal migration. Using precisely timed conditional knockout of Dab1 in migrating neurons, we found that Reelin signaling is essential only for a specific type of migration, called glia-independent somal translocation. While it is widely accepted in the field that disturbances in other modes of migration cause cortical lamination defects, these findings demonstrate that loss of glia-independent migration is equally disruptive. We further demonstrated that Reelin signaling promotes translocation of neurons by stabilizing their leading processes at the top of the cortex, by a mechanism involving Cadherin- and Nectin-dependent adhesions.
We also collaborate with other labs that study various aspects of neuronal migration and positioning.
Our lab has helped define the cellular mechanisms of Reelin function in cortical lamination and elucidate the molecular pathway downstream of Reelin signaling during neuronal migration. Using precisely timed conditional knockout of Dab1 in migrating neurons, we found that Reelin signaling is essential only for a specific type of migration, called glia-independent somal translocation. While it is widely accepted in the field that disturbances in other modes of migration cause cortical lamination defects, these findings demonstrate that loss of glia-independent migration is equally disruptive. We further demonstrated that Reelin signaling promotes translocation of neurons by stabilizing their leading processes at the top of the cortex, by a mechanism involving Cadherin- and Nectin-dependent adhesions.
We also collaborate with other labs that study various aspects of neuronal migration and positioning.
|
Neuron | (2011) Feb 10;69(3):482-97.
Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Developmental Neurobiology | (2011) Nov;71(11):889-900. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Franco SJ, Müller U. Cold Spring Harb Perspect Biol. | (2011) Jan 1;3(1):a005108. Extracellular matrix: functions in the nervous system. Barros CS, Franco SJ, Müller U. |
Elife | (2022) May 5;11:e76189
TUBA1A tubulinopathy mutants disrupt neuronal morphogenesis and override XMAP215/Stu2 regulation of microtubule dynamics Hoff KJ, Aiken JE, Gutierrez MA, Franco SJ, Moore JK. Development | (2016) June 15;143(12):2121-34. Cadherin 2/4 signaling via PTP1B and catenins is crucial for nucleokinesis during radial neuronal migration in the neocortex. Martinez-Garay I, Gil-Sanz C, Franco SJ, Espinosa A, Molnar Z, Mueller U. Neuron | (2013) Aug 7;79(3):461-77. Cajal-Retzius Cells Instruct Neuronal Migration by Coincidence Signaling between Secreted and Contact-Dependent Guidance Cues. Gil-Sanz C, Franco SJ, Martinez-Garay I, Espinosa A, Harkins-Perry S, Müller U. Glia | (2013) Aug;61(8):1347-63. Role of the postnatal radial glial scaffold for the development of the dentate gyrus as revealed by reelin signaling mutant mice. Brunne B, Franco S, Bouché E, Herz J, Howell BW, Pahle J, Müller U, May P, Frotscher M, Bock HH. |